Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.
If the pKa of CH3COOH and pKb of NH4OH are the same as 4.76, what is the pH of an aqueous solution of ammonium acetate? - Quora

Tell H3 â ‹QH-3 Calculate the percentage hydrolysis the pH of 0 02 M CH3COONH4 Kb(NH3) - Chemistry - Equilibrium - 12288037 | Meritnation.com

Assertion (A): An aqueous solution of ammonium acetate acts as a buffer solution. Reason (R) : A... - YouTube
![Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ] Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ]](https://d1hj4to4g9ba46.cloudfront.net/questions/1200266_999624_ans_e02120991ce8408c94c67fa9a8ab69a7.jpg)
Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ]
![Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ] Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ]](https://dwes9vv9u0550.cloudfront.net/images/9643998/9b1e3c5e-c251-4f47-8a31-4981f085e51d.jpg)
Calculate the extent of hydrolysis and the pH of 0.02 M CH3COONH4. [ Kb (NH3) = 1.8 × 10^- 5,Ka (COOH) = 1.8 × 10^- 5 ]

Chromatograms of drugs obtained using the suitable columns packed with... | Download Scientific Diagram

Ammonium acetate, Puriss. p.a., ACS Reagent, Reag. Ph. Eur., 98 , Honeywell Fluka | Fisher Scientific

WHICH OF THE FOLLOWING INCREASING ORDER OF PH OF .1 M SOLUTION OF THE COMPOUND A-HCOONH4,B-CH3COONH4 - Brainly.in
Identity correct statement (a)degree of hydrolysis decrease on doubling the concentration of aqueous solution of CH3COONH4 (b)for 1M CH3COOH pH=pKa/2 (c)salt hydrolysis depends on size of atom (d)all

Ammonium acetate which is 0.01 M, is hydrolysed to 0.001 M concentration. Calculate the change in pH - Brainly.in

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

Calculate the percentage hydrolysis & the pH 0.02 M CH3COONH4. Kb(NH3) = 1.6 × 10^-5, Ka(CH3COOH) = 1.6 × 10^-5.

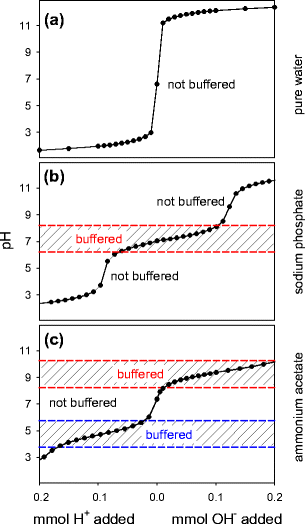
Addressing a Common Misconception: Ammonium Acetate as Neutral pH “Buffer” for Native Electrospray Mass Spectrometry | Journal of the American Society for Mass Spectrometry


![What is the pH of 0.02M CH3COONH4 ?[ Ka = 1.8 × 10^-5,Kb = 1.8 × 10^-5 ] What is the pH of 0.02M CH3COONH4 ?[ Ka = 1.8 × 10^-5,Kb = 1.8 × 10^-5 ]](https://haygot.s3.amazonaws.com/questions/1838174_1312507_ans_dfcdd24825da474ab196153f6eb7756a.jpg)