Analytical Methods - A Statistical Perspective On The ICH Q2A and Q2B Guidelines For Validation of Analytical Methods | PDF | Confidence Interval | Accuracy And Precision

Analytical Methods: A Statistical Perspective on the ICH Q2A and Q2B Guidelines for Validation of Analytical Methods - PDF Free Download
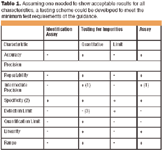
Analytical Methods: A Statistical Perspective on the ICH Q2A and Q2B Guidelines for Validation of Analytical Methods

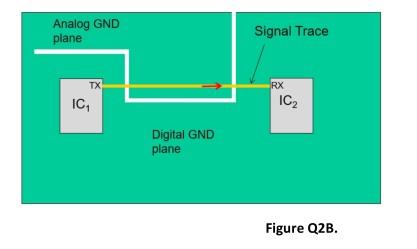
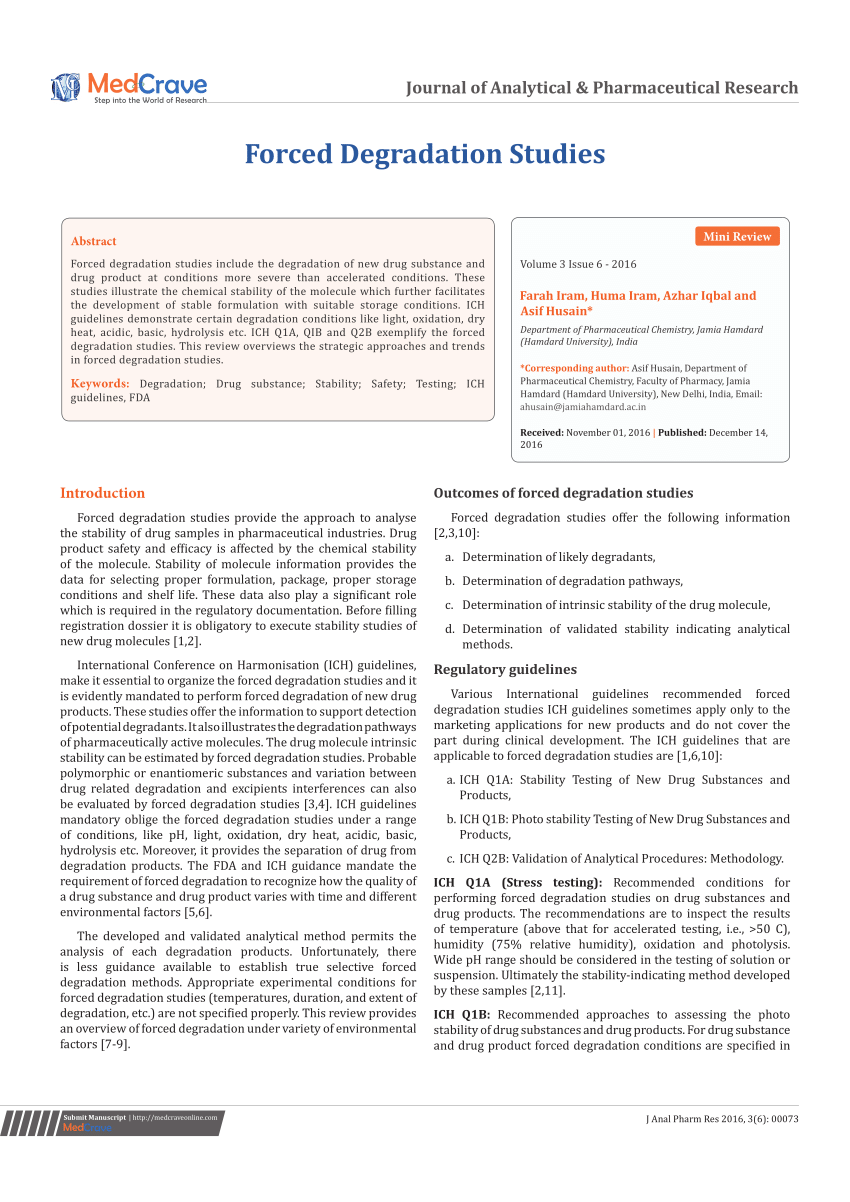
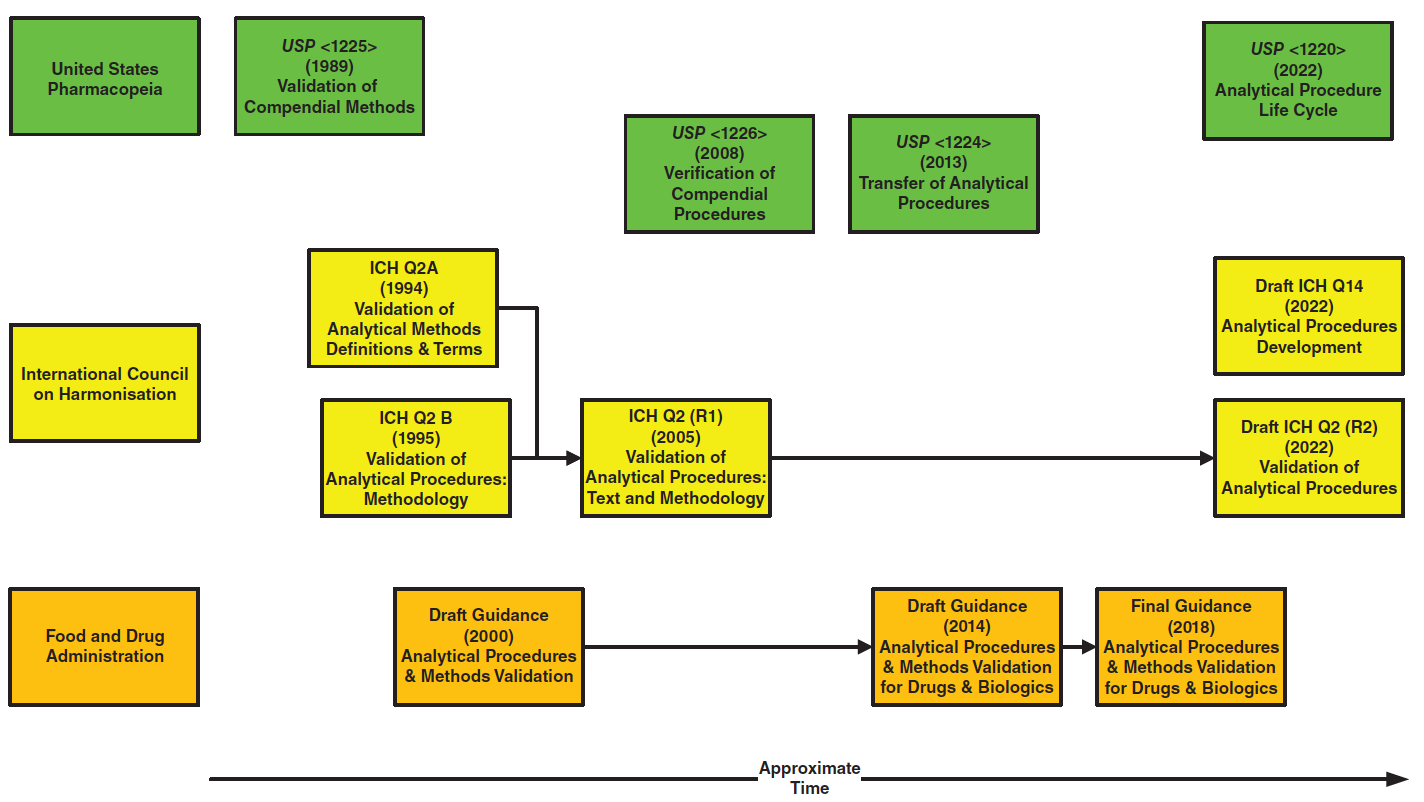
ICH Q2B Guideline Validation of Analytical Procedures Methodology - Bioanalytical Lab & Top CRO for Bioanalysis

Analytical Methods: A Statistical Perspective on the ICH Q2A and Q2B Guidelines for Validation of Analytical Methods
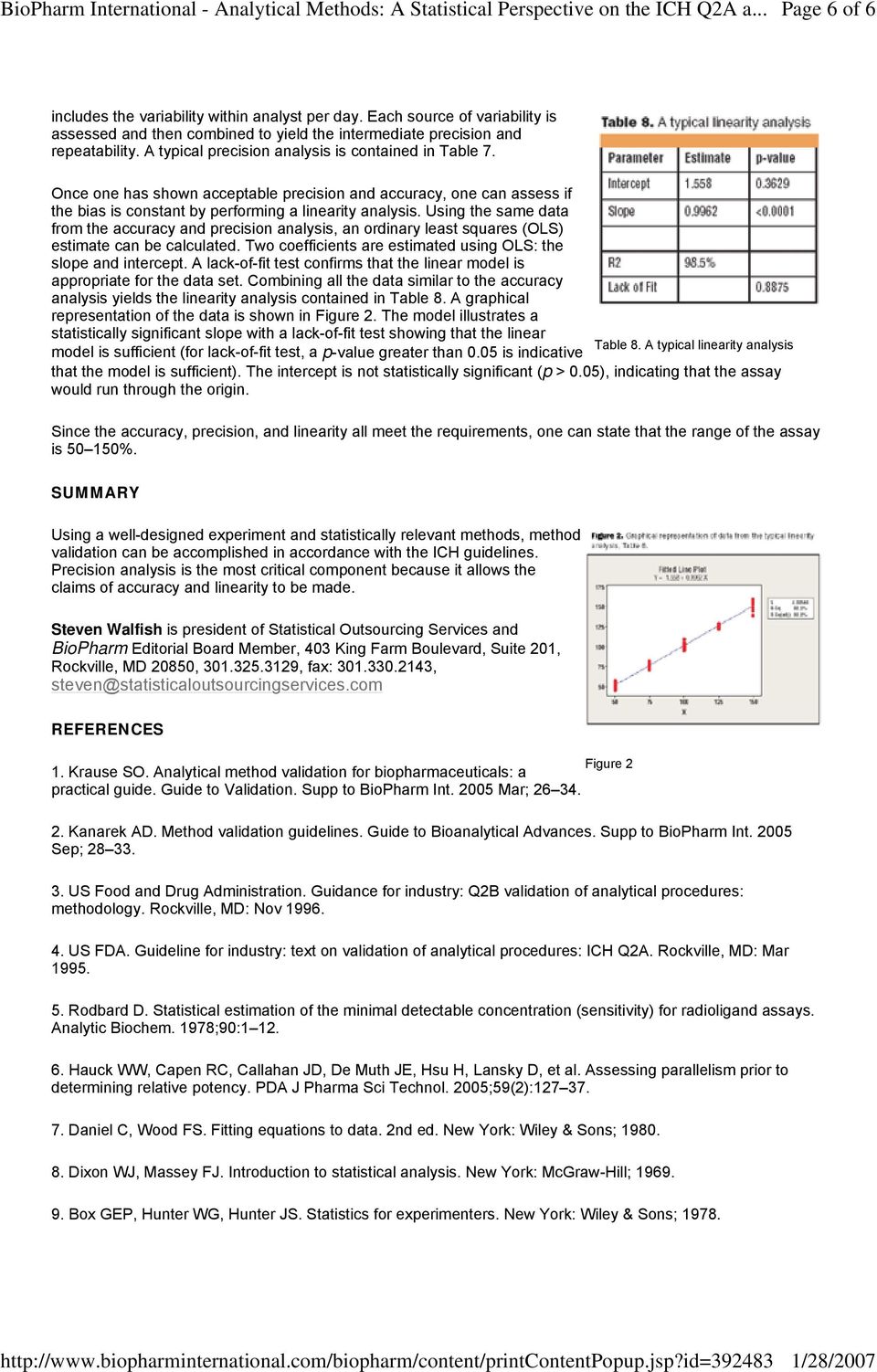
Analytical Methods: A Statistical Perspective on the ICH Q2A and Q2B Guidelines for Validation of Analytical Methods - PDF Free Download