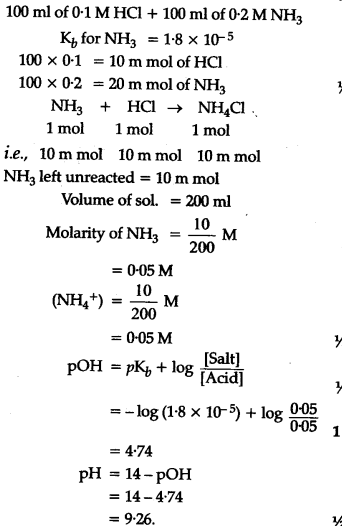
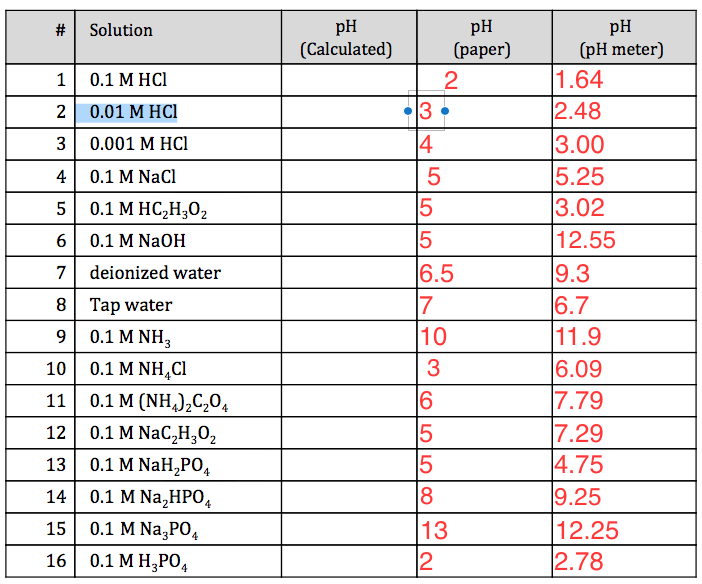
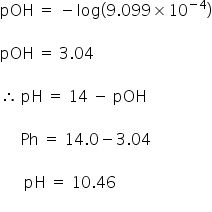
if equal volumes of 0.1 M H2SO4 and 0.1 M HCl are mixed then the pH of resulting solution will be (log 15 = 1.176)

Calculate the `pH` of a solution which contains `10 ml` of `1 M HCl` and `10 ml` of `2M NaOH` - YouTube
![50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ] 50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/OHZYS2ZrRHZKams=/sd/)
50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ]

a pH versus blank-corrected titration volume (0.1 M HCl) for PAAconf.b... | Download Scientific Diagram

SOLVED: 1. Calculate the pH if you added 3 mL of 0.1 M HCL to a) 97 mL of pure water at pH 7, and b) 100 mL of phosphate buffer (0.063

Calculate the pH of the resultant mixture: a. `10 mL` of `0.2M Ca(OH)_(2)+25 mL` of `0.1 M HCl` ... - YouTube

Calculate the pH of solution obtained by mixing 10 ml of 0.1M HCl and 40ml of 0.2 M H2SO4 - Brainly.in