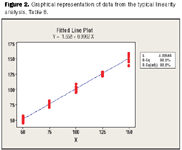
Analytical Methods: A Statistical Perspective on the ICH Q2A and Q2B Guidelines for Validation of Analytical Methods - PDF Free Download
Analytical Methods: A Statistical Perspective on the ICH Q2A and Q2B Guidelines for Validation of Analytical Methods

Analytical Methods: A Statistical Perspective on the ICH Q2A and Q2B Guidelines for Validation of Analytical Methods - PDF Free Download

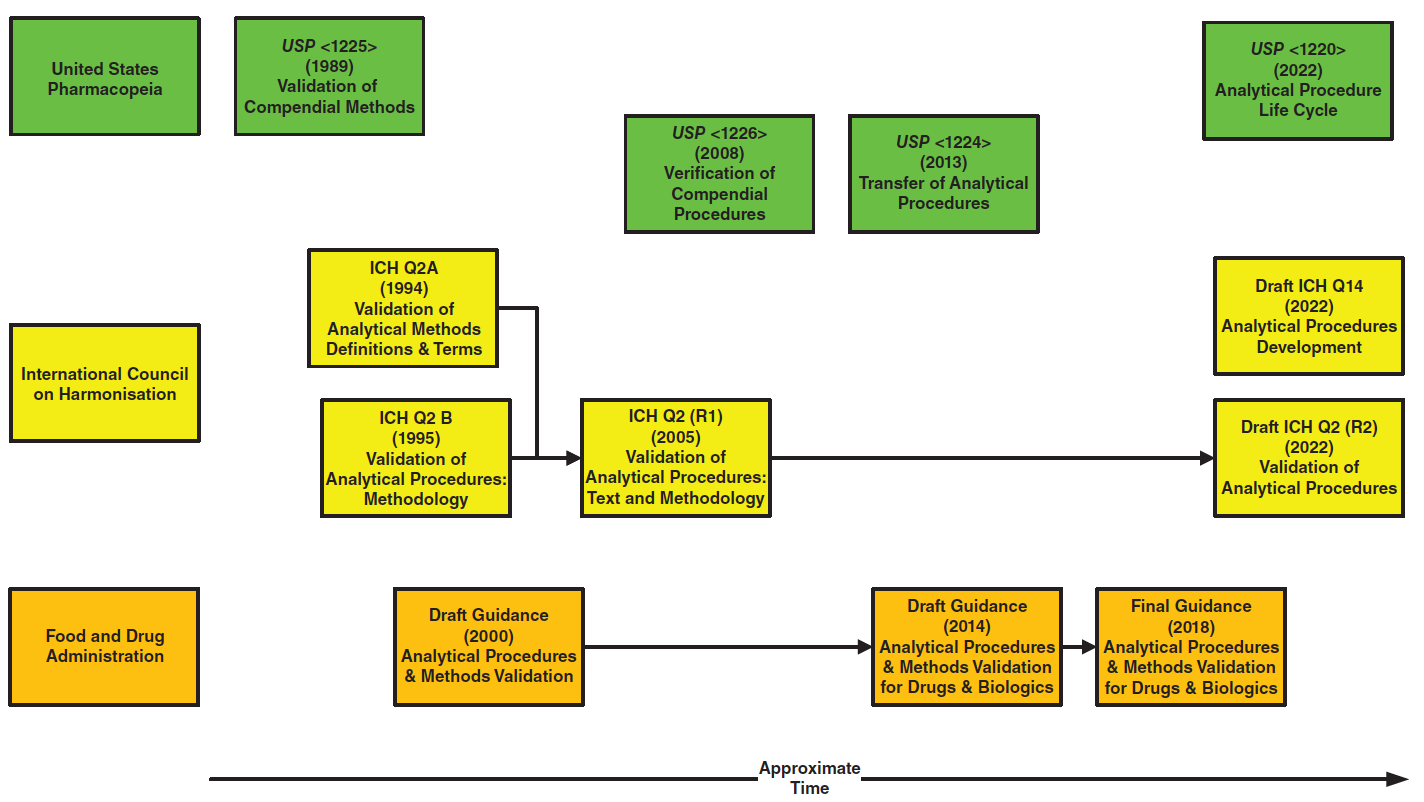
PDF) ICH Q2(R2)/Q14 Analytical Procedure Validation and Development€¦ · Objectives ICH Q2(R1) Revision • Provide a general framework for the principles of analytical procedure validation - DOKUMEN.TIPS