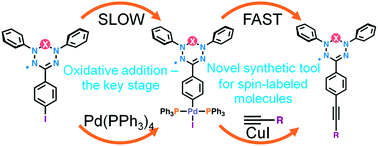
Pd(PPh3)4-PEG 400 Catalyzed Protocol for the Atom-Efficient Stille Cross-Coupling Reaction of Organotin with Aryl Bromides
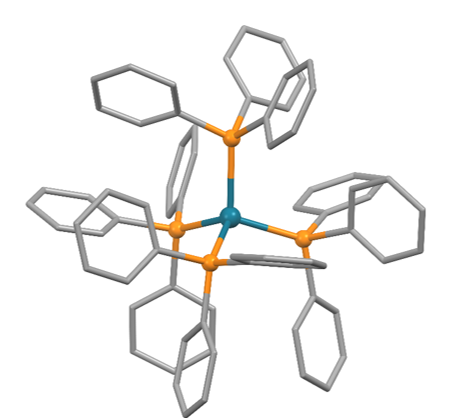
![Molecules | Free Full-Text | Syntheses, Reactivities, Characterization, and Crystal Structures of Dipalladium Complexes Containing the 1,3-pyrimidinyl Ligand: Structures of [Pd(PPh3)(Br)]2(μ,η2-C4H3N2)2, [Pd(Br)]2(μ,η2-Hdppa)2, and [{Pd(PPh3)(CH3CN)}2 ... Molecules | Free Full-Text | Syntheses, Reactivities, Characterization, and Crystal Structures of Dipalladium Complexes Containing the 1,3-pyrimidinyl Ligand: Structures of [Pd(PPh3)(Br)]2(μ,η2-C4H3N2)2, [Pd(Br)]2(μ,η2-Hdppa)2, and [{Pd(PPh3)(CH3CN)}2 ...](https://www.mdpi.com/molecules/molecules-25-02035/article_deploy/html/images/molecules-25-02035-g001.png)
Molecules | Free Full-Text | Syntheses, Reactivities, Characterization, and Crystal Structures of Dipalladium Complexes Containing the 1,3-pyrimidinyl Ligand: Structures of [Pd(PPh3)(Br)]2(μ,η2-C4H3N2)2, [Pd(Br)]2(μ,η2-Hdppa)2, and [{Pd(PPh3)(CH3CN)}2 ...

Reactions of Pd(PPh3)4 with 3',5'-Di-O-acetylthymidine: Oxidative Addition of Pd(PPh3)4 on Thymidine N3 and C4 Atoms | Organometallics

Reagents and conditions: (i) PhCCH, CuI, Et3N, Pd(PPh3)4, DMF, 80°C,... | Download Scientific Diagram
![Reactivity of a Dinuclear PdI Complex [Pd2(μ-PPh2)(μ2-OAc)(PPh3)2] with PPh3: Implications for Cross-Coupling Catalysis Using the Ubiquitous Pd(OAc)2/nPPh3 Catalyst System | Organometallics Reactivity of a Dinuclear PdI Complex [Pd2(μ-PPh2)(μ2-OAc)(PPh3)2] with PPh3: Implications for Cross-Coupling Catalysis Using the Ubiquitous Pd(OAc)2/nPPh3 Catalyst System | Organometallics](https://pubs.acs.org/cms/10.1021/acs.organomet.1c00347/asset/images/large/om1c00347_0011.jpeg)
Reactivity of a Dinuclear PdI Complex [Pd2(μ-PPh2)(μ2-OAc)(PPh3)2] with PPh3: Implications for Cross-Coupling Catalysis Using the Ubiquitous Pd(OAc)2/nPPh3 Catalyst System | Organometallics

An Improved Catalytic System, Pd(PPh3)4/PhCOOH Combined Catalyst, for the Allylation of Carbon Pronucleophiles with Allenes

China Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) CAS No.: 14221-01-3 Manufacturers - Free Sample - Alfa Chemical

An Improved Catalytic System, Pd(PPh3)4/PhCOOH Combined Catalyst, for the Allylation of Carbon Pronucleophiles with Allenes
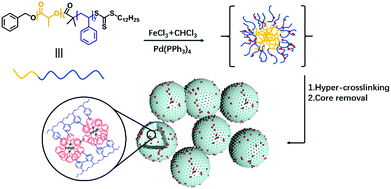
Hollow porous organic nanospheres for anchoring Pd(PPh3)4 through a co-hyper-crosslinking mediated self-assembly strategy - New Journal of Chemistry (RSC Publishing)

Scheme 1. Preparation of pyrimidines 1 and 2. (i) Toluene, Pd(PPh3)4,... | Download Scientific Diagram

Pd(PPh3)4‐Catalyzed Buchwald–Hartwig Amination ofAryl Fluorosulfonates with Aryl Amines - Lim - 2017 - Asian Journal of Organic Chemistry - Wiley Online Library
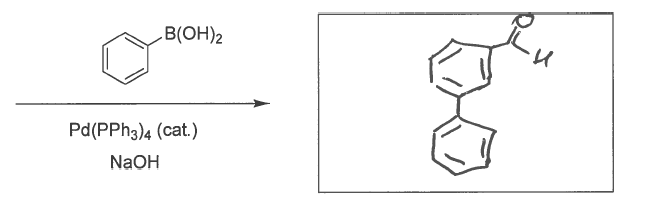
![Nuances in Fundamental Suzuki–Miyaura Cross-Couplings Employing [Pd(PPh3)4]: Poor Reactivity of Aryl Iodides at Lower Temperatures | Organometallics Nuances in Fundamental Suzuki–Miyaura Cross-Couplings Employing [Pd(PPh3)4]: Poor Reactivity of Aryl Iodides at Lower Temperatures | Organometallics](https://pubs.acs.org/cms/10.1021/acs.organomet.8b00189/asset/images/medium/om-2018-00189e_0001.gif)

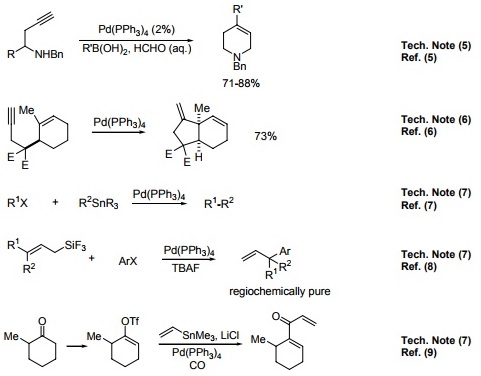
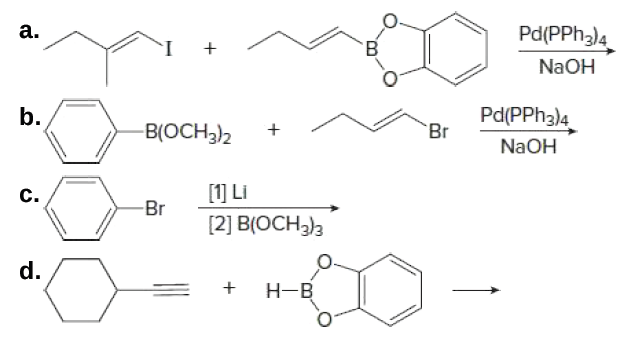

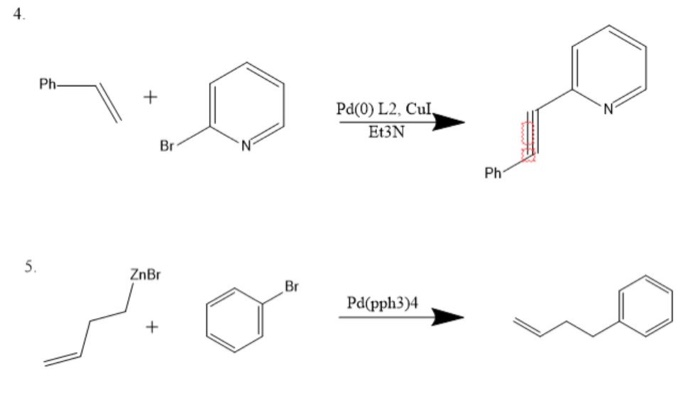
![Solved (c) [Pd(PPh3)4] and [Pd(PPh3)2Cl2] are common | Chegg.com Solved (c) [Pd(PPh3)4] and [Pd(PPh3)2Cl2] are common | Chegg.com](https://media.cheggcdn.com/media/018/018b9260-7979-456f-90d3-abf32cfbe911/phpbOUnpK)