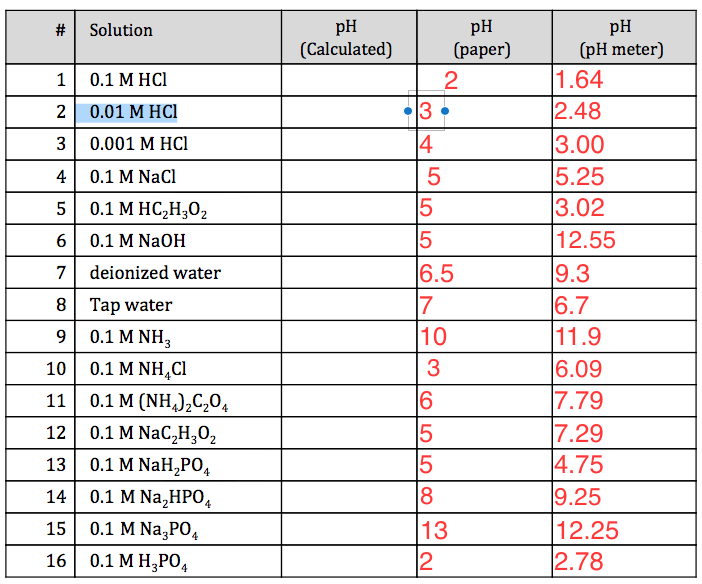
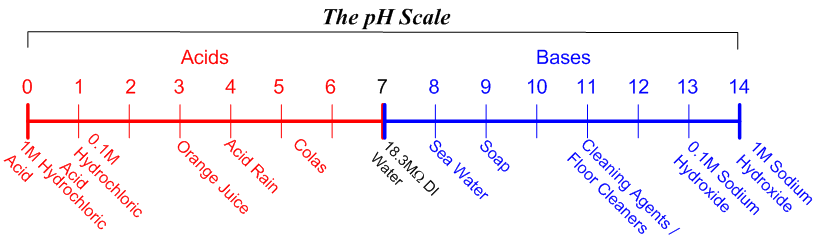
What is the pH of a solution containing 50ml of 1M HCl and 100ml of 0 25M HCl Answer - Chemistry - Equilibrium - 13180741 | Meritnation.com

The changes in pH after additon of various volumes of 1M HCl and 1M... | Download Scientific Diagram