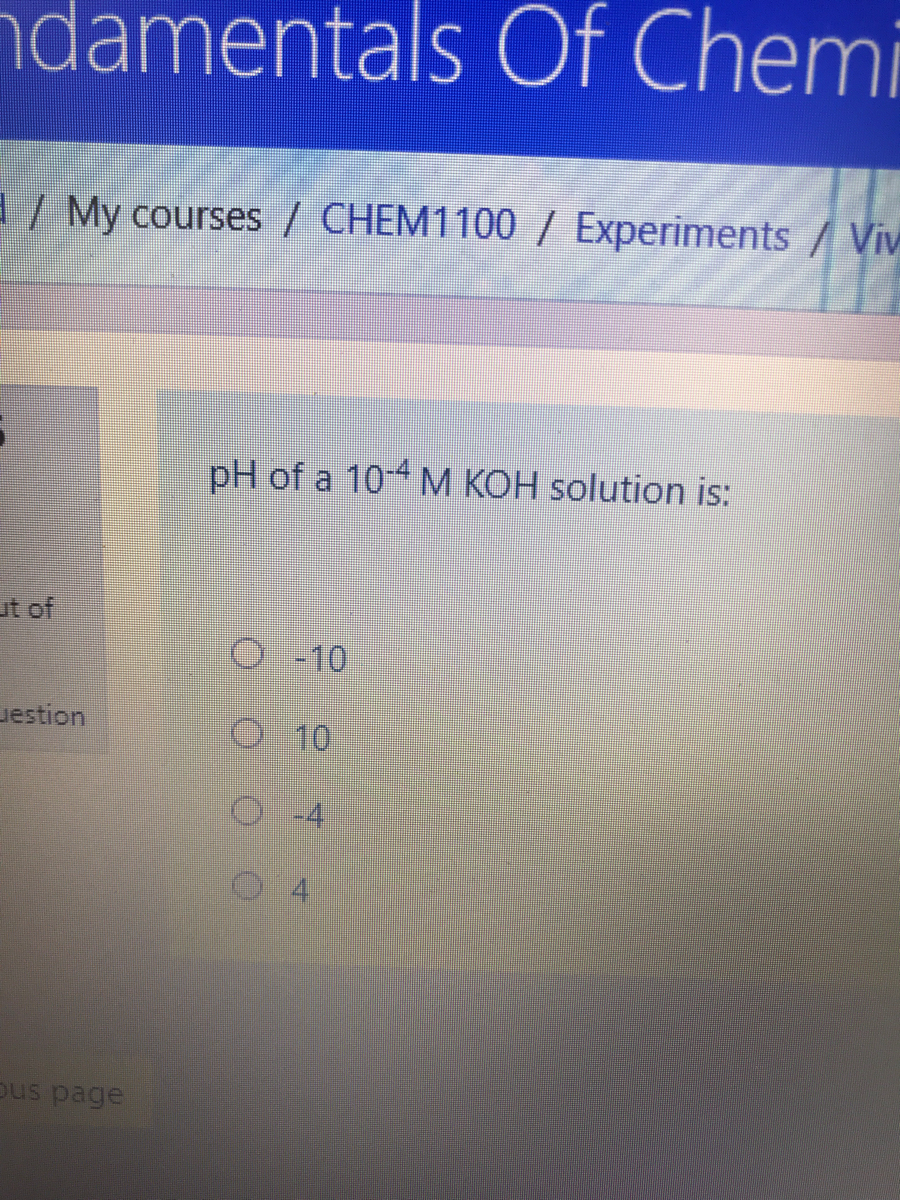
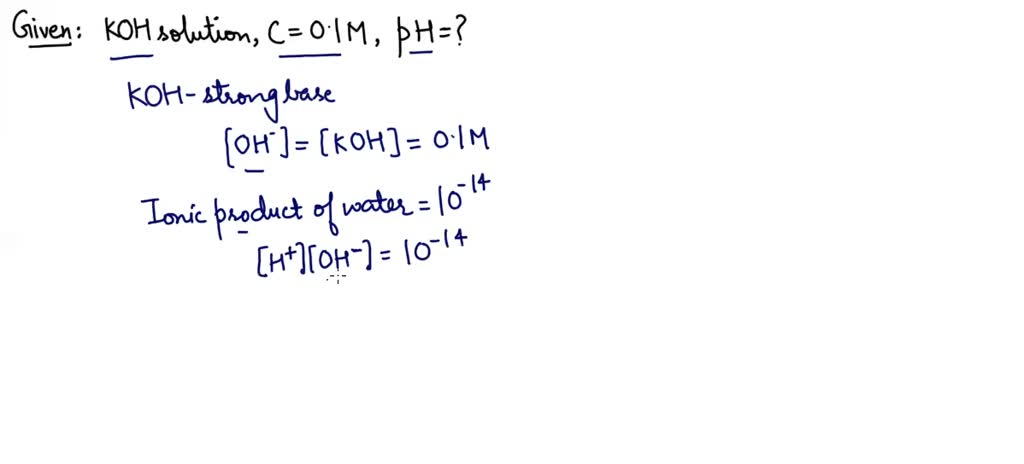SOLVED: Problem Solving: What are the pH values of (a) 2.0 × 10−2 mol/L KOH and of (b) 2.0 × 10−6 mol/L KOH?

Koh asit mi baz mı, yaygın adı nedir ve kuvvetli baz mıdır? Potasyum hidroksit Ph değeri ve kullanım alanları

Consider the titration of 0.300 L of 0.200 M KOH with HI, the results of which are shown in the following curve: pH 7.0 150 mL acid What is the concentration of

50 ml 01m koh is mixed with 50 ml 01m hcooh ph of the final solution is 6bh2onss -Chemistry - TopperLearning.com
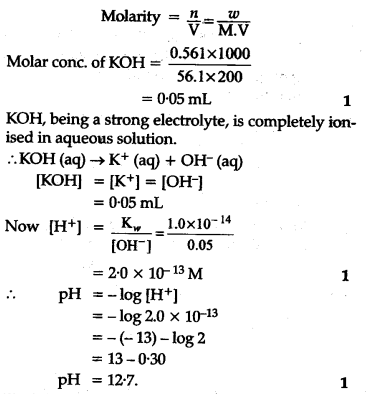
If 0-561 g of KOH is dissolved in water to give 200 mL of solution at 298 K. Calculate the concentrations of potassium, hydrogen and hydroxyl ions. What is its pH? -
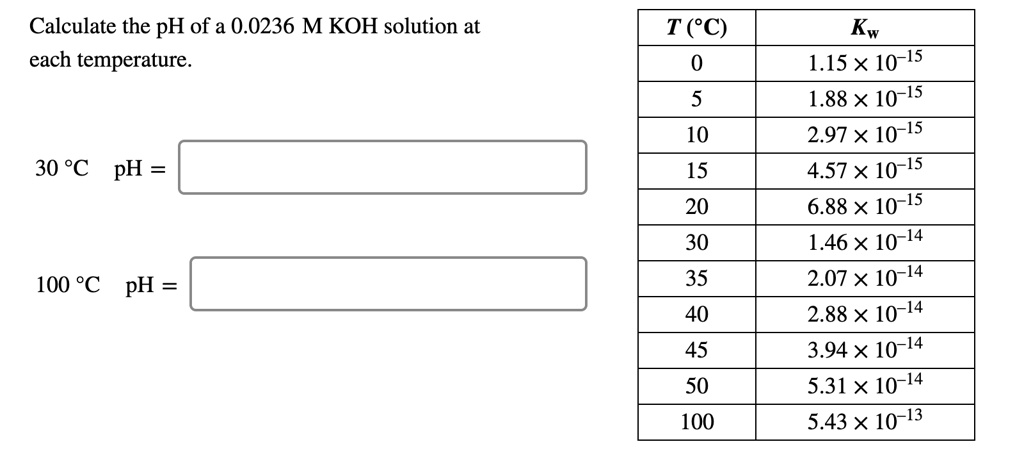
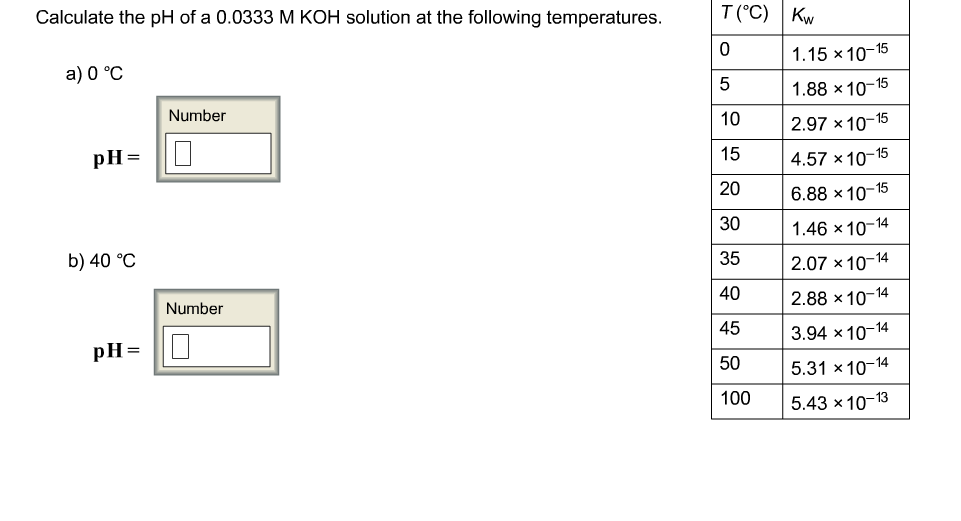
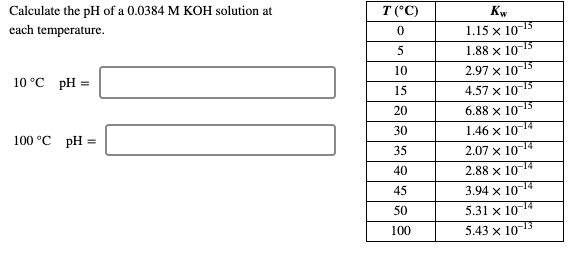
SOLVED: Calculate the pH of a 0.0236 M KOH solution at each temperature. T(C) Kw 1.15 X 10-15 1.88 X 10-15 2.97 X 10-15 4.57X 10-15 6.88 X 10-15 1.46 X 10-14 2.07 X 10-14 2.88 X 10-14 3.94 X 10-14 5.31X 10-14 5.43X 10-13 10 15 20 30 35 40 45 50 100 30 ...

The `pH` of `0.01 (M) KOH` is `12`, if the temperature of the given `KOH` solution is increased - YouTube
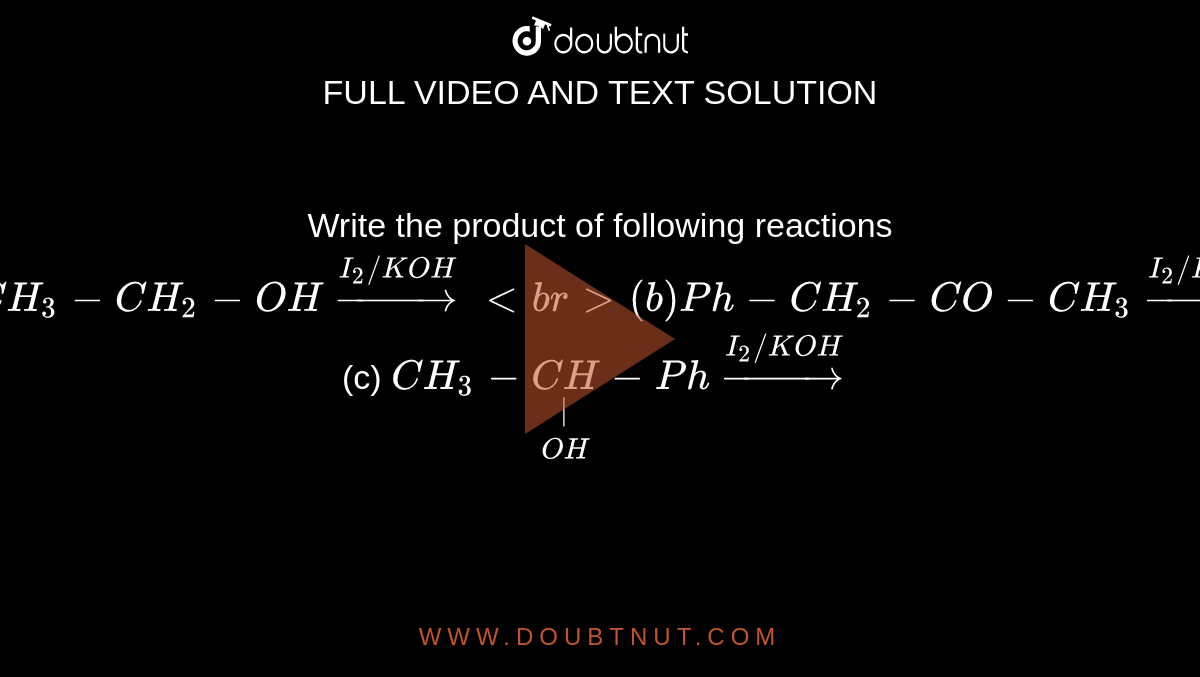
Write the product of following reactions (a) CH3-CH(2)-OH overset(I(2)//KOH)to (b)Ph-CH(2)-CO-CH(3)overset(I(2)//KOH)to (c) CH(3)-underset(OH)underset(|)(CH)-Ph overset(I(2)//KOH)to